Abstract
INTRODUCTION: Thrombotic events are common in the course of COVID-19 and are related to the release of neutrophil extracellular traps (NETs). Identifying the moment during the time course of the disease when the stimulus for NETs and platelet activation occurs and finding potential drugs that inhibit these mechanisms will bring insights on optimal time to initiate treatment.
AIM: To evaluate changes in the levels of inflammatory, platelet activation and NETs markers during COVID-19 hospitalization and their association with adverse outcomes. We also analyzed whether antiplatelets and anticoagulants were capable of inhibiting NETs release "in vitro".
METHODS: First we quantified circulating levels of IL-8, IL-6, TNF-α, RANTES and citrullinated histone H3 (cit-H3) on four occasions during COVID-19 hospitalization (admission, day 4, day 8 and end of hospitalization) between May and July 2020 and we assessed their association with clinical outcomes. Second, we stimulated healthy neutrophils (20x103/well) with phorbol myristate acetate (PMA) or serum from severe COVID-19 patients (n=15) in the presence of heparin (25ug/mL, 50ug/mL and 100ug/mL), enoxaparin (25ug/mL, 50ug/mL and 100ug/mL), aspirin (1mM, 2.5mM and 5mM) or ticagrelor (1uM, 2.5uM and 5uM) and in the absence of these drugs. NETs formation was quantified using Incucyte. Neutrophils nuclei were stained with NuclearRed ID and the cells were incubated with a medium containing Sytox Green. Images of the cells incubation were obtained after 1.5 hour, the number of cells that suffered netosis (stained in green) and did not suffer (stained in red) was obtained. Finally, we calculated the percentage of netosis dividing the number of green cells by the total number of cells per well.
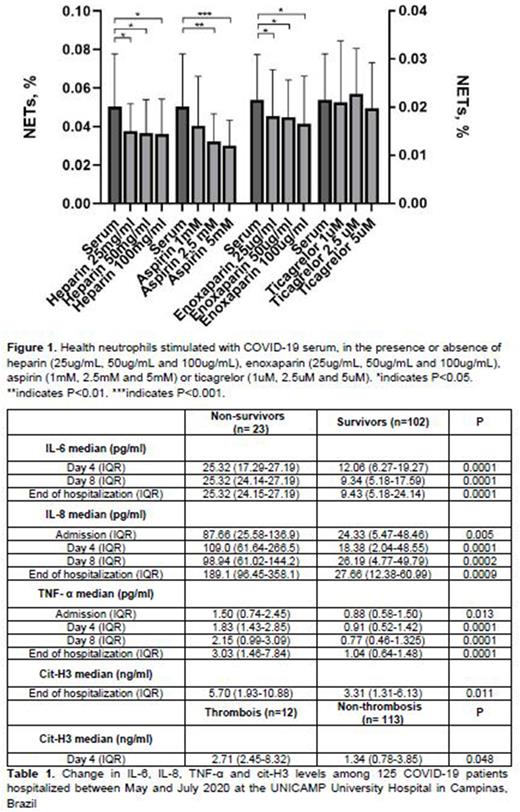
RESULTS: Table 1 illustrates the changes in IL-8, IL-6, TNF-α, cit-H3 and RANTES levels during the COVID-19 hospitalization. IL-6 levels were higher in non-survivors than in survivors from day 4 to the end of hospitalization. IL-6 levels remained unchanged from admission until the end of hospitalization in non-survivors while decreased in survivors. IL-8 levels were higher in non-survivors than in survivors during the entire hospitalization period. TNF-α levels remained higher in non-survivor than in survivors during the entire hospitalization. CitH3 levels were similar between non-survivors and survivors until day 8, but were 2-fold higher in non-survivors at the end of hospitalization. While IL-6, IL-8 and TNF-α levels were not associated with thrombotic events, citH3 levels were 2-fold higher on day 4 of hospitalization in patients who had thrombosis than in those without this complication, shortly before the occurrence of the thrombotic event. RANTES levels were similar between survivors and non-survivors and were not associated with thrombosis. Figure 1 illustrates the potential of NETs released after neutrophils being stimulated with COVID-19 serum in the presence and absence of the four drugs tested. The percentage of COVID-19-induced NETs were higher in non-treated neutrophils than in pretreated neutrophils with heparin and enoxaparin at all 3 concentrations (25ug/mL, 50ug/mL and 100ug/mL). Aspirin was capable of modulating COVID-19 induced netosis at the concentrations of 2.5mM and 5mM, but not at 1mM. A similar effect was observed in the PMA-19-induced netosis. Heparin and enoxaparin also decreased netosis at the 3 concentrations after PMA stimulus, while aspirin reduced netosis induced by PMA only at 2.5mM and 5mM. Ticagrelor showed no effect on either COVID-19 or PMA induced NETs release.
CONCLUSION: IL-6, IL-8, TNF-α and cit-H3 levels were related to poor clinical outcomes in COVID-19 patients during the time course of the disease. Since the beginning of the hospitalization, an increased inflammatory response was observed, while markers of NETs arose later. Heparin, enoxaparin and aspirin were capable of "in vitro'' modulating netosis induced by COVID-19 and PMA in different concentrations. Such findings suggest that thromboinflammatory stimulus occurs later during COVID-19 clinical course and provide evidence on possible effects of heparin and aspirin on modulating NETs formation.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal